SMO
SMO
SMO
Shared Manufacturing Organization
Shared Manufacturing Organization
Shared Manufacturing Organization


A bioproduction service at your disposal
A bioproduction service at your disposal
A bioproduction service at your disposal
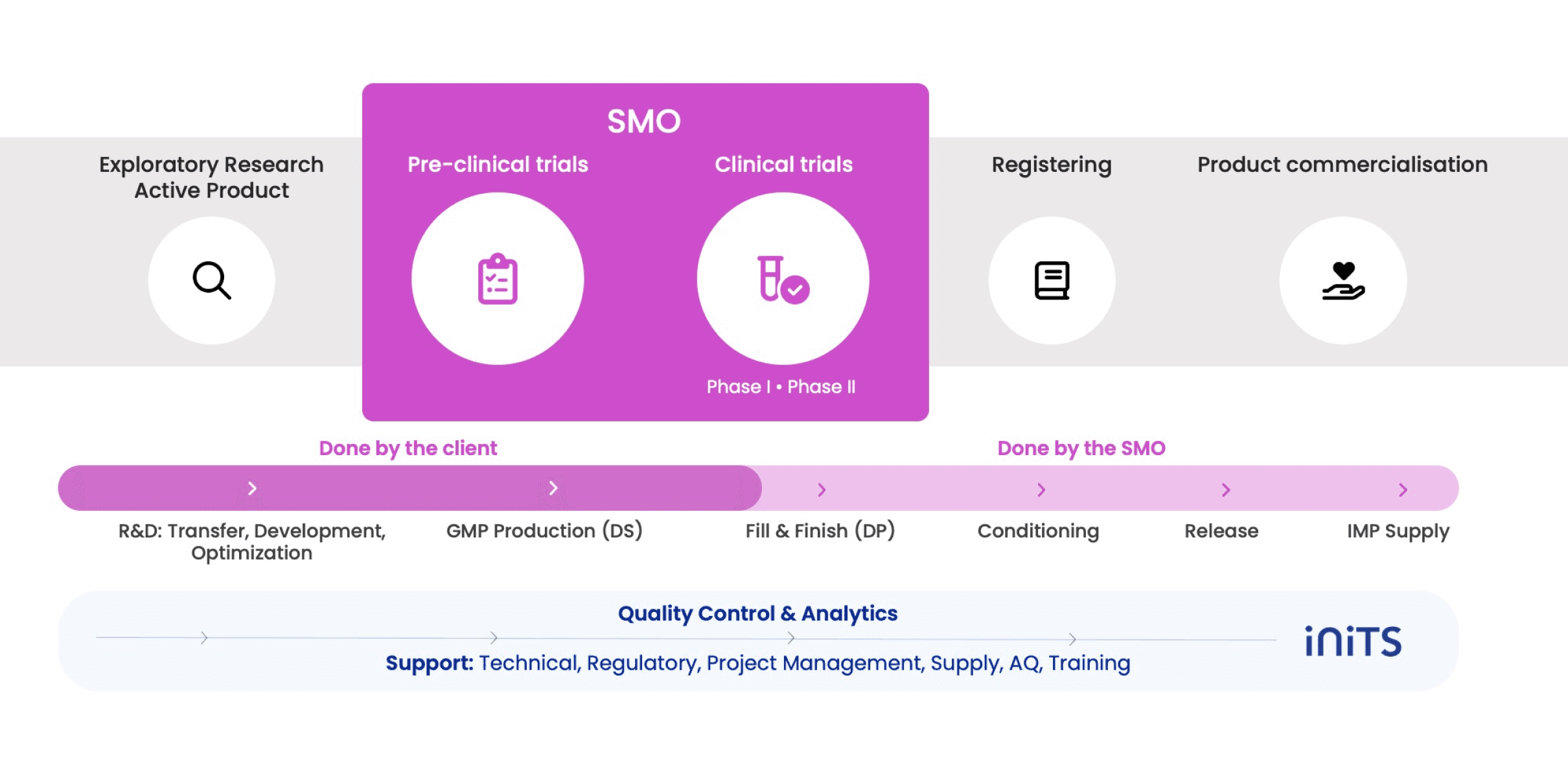
A disruptive model where the partner keeps the control. Our flexible biomanufacturing unit is multiproducts, allowing customers to produce in a controlled and transparent environment from R&D to GMP production.
Inits SMO allows you to keep the know how by producing yourself in our DS GMP facilities while benefiting from INITS’ expertise support (QA, production, QC).
We offer a fully integrated service with DP production, QP release and IMP supply, performed by Inits SMO.
A disruptive model where the partner keeps the control. Our flexible biomanufacturing unit is multiproducts, allowing customers to produce in a controlled and transparent environment from R&D to GMP production.
Inits SMO allows you to keep the know how by producing yourself in our DS GMP facilities while benefiting from INITS’ expertise support (QA, production, QC).
We offer a fully integrated service with DP production, QP release and IMP supply, performed by Inits SMO.
A disruptive model where the partner keeps the control. Our flexible biomanufacturing unit is multiproducts, allowing customers to produce in a controlled and transparent environment from R&D to GMP production.
Inits SMO allows you to keep the know how by producing yourself in our DS GMP facilities while benefiting from INITS’ expertise support (QA, production, QC).
We offer a fully integrated service with DP production, QP release and IMP supply, performed by Inits SMO.
Benefits
Benefits
Benefits
A bioproduction service at your disposal
A bioproduction service at your disposal
A bioproduction service at your disposal
Flexible, multiproducts with modern and high-tech tools
Flexible, multiproducts with modern
and high-tech tools
Flexible, multiproducts with modern and high-tech tools
Keep control over the process
Keep control over the process
Keep control over the process
A fully integrated service provider
A fully integrated service provider
A fully integrated service provider


The perfect environment for your product development
The perfect environment for your product development
The perfect environment for your product development
We offer a way for our clients to keep control over their product development.
We offer a way for our clients to keep control over their product development.
3200
3200
3200
Square meter
of spaces
Square meter
of spaces
Square meter
of spaces
3
3
3
R&D production suites
with client office spaces
R&D production suites
with client office spaces
R&D production suites
with client office spaces
4
4
4
GMP production suites with client office spaces
GMP production suites with client office spaces
GMP production suites with client office spaces
1
1
1
Fill & Finish GMP suite
Fill & Finish GMP suite
Fill & Finish GMP suite
3
3
3
Quality Control laboratories
Quality Control laboratories
Quality Control laboratories
1
1
1
Packaging suite
Packaging suite
Packaging suite



Fully-equipped GMP Suites
Fully-equipped GMP Suites
Fully-equipped GMP Suites
1 USP/DSP (fully-equiped, class C)
Incubators, 50L + 200L bioreactors, chromatography system, TFF system
1 USP/DSP (fully-equiped, class C)
incubators, 50L + 200L bioreactors, chromatography system, TFF system
1 USP/DSP (fully-equiped, class C)
Incubators, 50L + 200L bioreactors, chromatography system, TFF system
1 with no equipment, class C
1 with no equipment, class C
1 with no equipment, class C
1 USP/DSP (fully-equiped, class C)
200L bioreactor, chromatography system, TFF system
1 USP/DSP (fully-equiped, class C)
200L bioreactor, chromatography system, TFF system
1 USP/DSP (fully-equiped, class C)
200L bioreactor, chromatography system, TFF system
Fill & Finish suite with an automatic filling in class A isolator
Fill & Finish suite with an automatic filling in class A isolator.
Fill & Finish suite with an automatic filling in class A isolator.
1 for cell culture and TC class A in B
Incubators, prodigy
1 for cell culture and TC class A in B
incubators, prodigy
1 for cell culture and TC class A in B
Incubators, prodigy
R&D Suites
R&D Suites
R&D Suites
1 USP/DSP (fully-equiped)
Incubators, 2L + 10L + 50L bioreactors, chromatography system, TFF system
1 USP/DSP (fully-equiped)
Incubators, 2L + 10L + 50L bioreactors, chromatography system, TFF system
1 USP/DSP (fully-equiped)
Incubators, 2L + 10L + 50L bioreactors, chromatography system, TFF system
1 for cell culture and TC
Incubators, prodigy
1 for cell culture and TC
Incubators, prodigy
1 for cell culture and TC
Incubators, prodigy
1 with no equipment
QC Labs
QC Labs
QC Labs
1 for microbiology
1 for microbiology
1 for cell culture
1 for cell culture
1 for biochemistry
1 for biochemistry
Labelling, Packaging and QP Release
Labelling, Packaging and QP Release
Labelling, Packaging and QP Release
Visual Inspection Suite
Visual Inspection Suite
Visual Inspection Suite
Edition Suite
Edition Suite
Edition Suite
Packaging Suite
Packaging Suite
Packaging Suite
Sampling Suite
Sampling Suite
Sampling Suite
We offer a wide range of In-House quality controls tests.
We offer a wide range of In-House quality controls tests.
We offer a wide range of In-House quality controls tests.
Microbiology, cell assays, biochemistry and molecular biology
Microbiology, cell assays, biochemistry and molecular biology
Microbiology, cell assays, biochemistry and molecular biology
We work with qualified subcontractors for other assays.
We work with qualified subcontractors for other assays.
We work with qualified subcontractors for other assays.
Our expertise and support
Our expertise and support
Our expertise and support
INITS SMO will provide its expertise throughout this process to cover all aspects necessary to produce compliant drugs: regulatory, quality assurance, quality control, supply management, project management.
INITS SMO will provide its expertise throughout this process to cover all aspects necessary to produce compliant drugs: regulatory, quality assurance, quality control, supply management, project management.
INITS SMO will provide its expertise throughout this process to cover all aspects necessary to produce compliant drugs: regulatory, quality assurance, quality control, supply management, project management.






Easily accessible
Easily accessible
Easily accessible
Close to Montpellier’s airport and train station.
Close to Montpellier’s airport and train station.




For inquiries or to explore how our services can benefit your specific needs
For inquiries or to explore how our services can benefit your specific needs
For inquiries or to explore how our services can benefit your specific needs
© 2024 INITS. All rights reserved
© 2024 INITS. All rights reserved
© 2024 INITS. All rights reserved
